The BEAD Feasibility Study
Does the baby head elevation cushion (Fetal Pillow®) make an emergency caesarean section, when the cervix is fully dilated, safer for māmā and pēpi?
BEAD study video – English
BEAD study video – te reo Māori
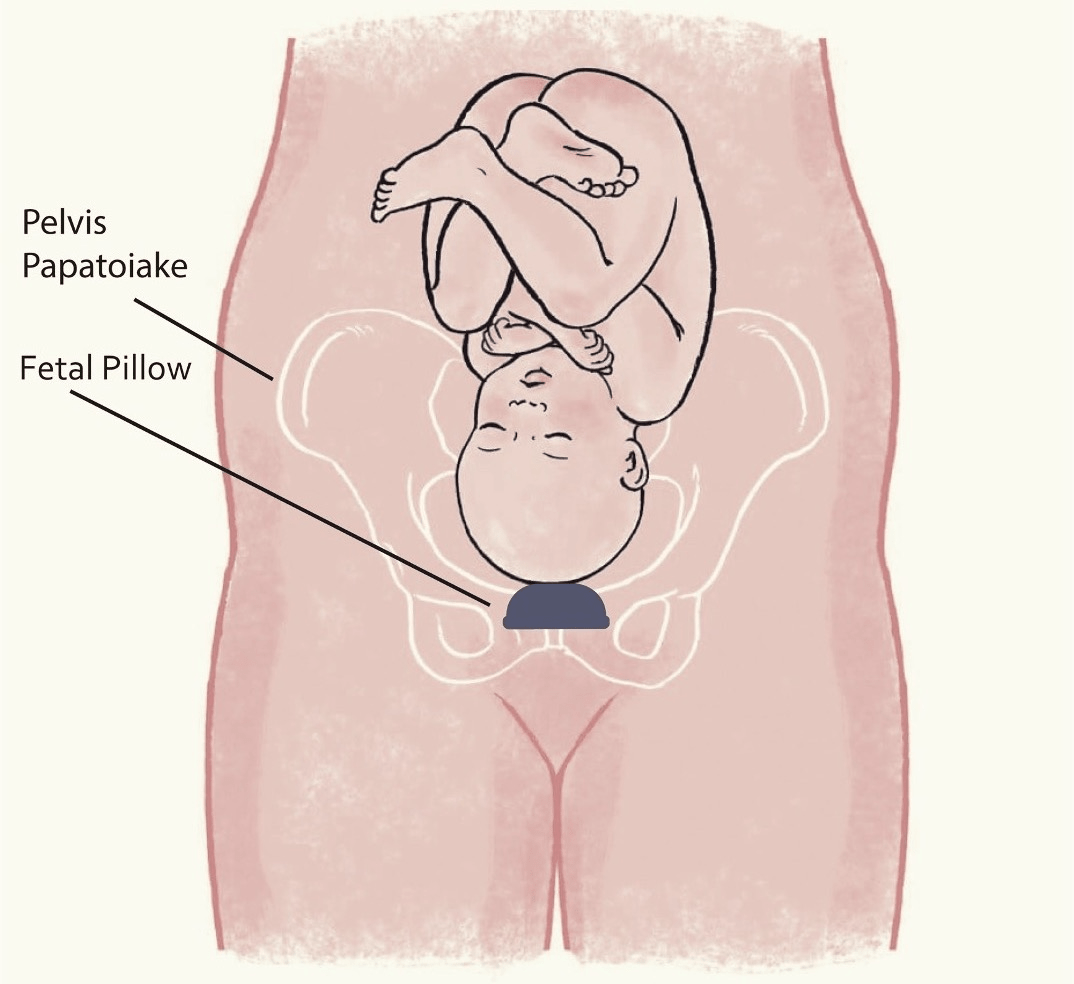
What is a Fetal Pillow®?
The Fetal Pillow® is a small, soft silicone balloon that is placed in the vagina before the caesarean and many doctors have started to use it. The balloon aims to elevate the baby’s head to make the caesarean section easier.
What is the BEAD Feasibility Study?
This study will increase our understanding of women and whānau experiences of using the baby head elevation cushion (Fetal Pillow®). We want to learn about any challenges or barriers to people taking part, at the time of labour and birth. This study will tell us how best to undertake a future similar larger study.
Why is the BEAD Feasibility Study important?
In Aotearoa New Zealand around 1500 birthing people (2-3% of all births) have an emergency caesarean section at full dilatation (10cm) each year. Caesarean section at this stage of labour is associated with increased risks such as injury to the uterus, resulting in longer operating time and increased blood loss. This may be due to difficulty with delivering the baby’s head which can be deep in the pelvis. We also know that having a caesarean section at full dilatation increases the risk of having a preterm birth in a future pregnancy. We want to know whether using the Fetal Pillow® safely reduces these risks.
Why is the BEAD Feasibility Study important?
In Aotearoa New Zealand around 1500 birthing people (2-3% of all births) have an emergency caesarean section at full dilatation (10cm) each year. Caesarean section at this stage of labour is associated with increased risks such as injury to the uterus, resulting in longer operating time and increased blood loss. This may be due to difficulty with delivering the baby’s head which can be deep in the pelvis. We also know that having a caesarean section at full dilatation increases the risk of having a preterm birth in a future pregnancy. We want to know whether using the Fetal Pillow® safely reduces these risks.
Where will the study take place?
At two hospitals in Auckland, New Zealand over a 12 month period:
- Te Toka Tumai Auckland (Auckland City Hospital)
- Te Whatu Ora Counties Manukau (Middlemore Hospital)
Who can take part?
If you are pregnant with one baby that is head down (cephalic) and you are more than 37 weeks’ pregnant and need an emergency caesarean section when you are fully dilated.
This study may not be suitable if your baby is unwell, you are having twins or triplets, are less than 37 weeks’ pregnant or have a baby that is not head down (e.g. breech).
Please let your midwife or obstetrician know if you would like to take part or contact the BEAD study team to let them know you are interested.
What is involved?
If you are interested, we will give you a participant information sheet and consent form to read before your caesarean section.
If you are eligible and agree to take part, you will have the Fetal Pillow® placed in the vagina (by your surgeon) immediately prior to your emergency caesarean section at full dilatation. This will either be inflated (with water) or not inflated. The anaesthetist (who is in charge of making you comfortable during the operation) will be responsible for the Fetal Pillow® being inflated or not inflated. You, your midwife and your surgeon won’t know if the balloon has been inflated and you can’t choose which group you are in.
After your caesarean, a member of our team will visit you while you are in hospital and ask your consent to collect information about you and your baby.
All eligible birthing women who agreed to participate in the study or not will be invited to complete a simple survey to help increase our understanding for future Fetal Pillow® research.
Are there any risks?
The Fetal Pillow® is already used in many maternity hospitals across New Zealand. Previous international studies have not shown any harms to mothers or babies from its use. This study is designed to assess the benefits (and risks) of using the Fetal Pillow® with the aim to improve maternity care in the future.
Who is organising the study?
The BEAD Feasibility Study is led by:

Jordon Wimsett

Robin Cronin

John Thompson

Lynn Sadler

Charlotte Oyston
Matthew Drake

Jane Alsweiler

Meghan Hill

Karaponi Okesene-Gafa

Erena Browne
Who is funding the study?
The BEAD Feasibility Study is funded by grants from the Health Research Council of New Zealand and the Mercia Barnes Trust.
Find out more
Contact the BEAD Study Research Coordinator via email at: thebeadstudy@auckland.ac.nz
- Auckland City Hospital, Dr. Lynn Sadler
- Middlemore Hospital, Dr. Charlotte Oyston